
|
Photophysics and Photochemistry of Transition Metal Compounds |
| Home Research Members Collaborations Publications |

|
 |
|||||||
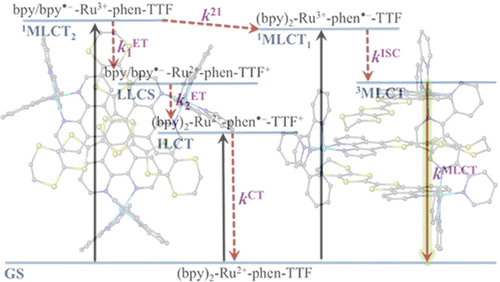
Ru(II) complexes with chelating ligands, 4′,5′-ethylenedithiotetrathiafulvenyl[4,5-f][1,10]phenanthroline (L1), 1,3-dithiole-2-thiono[4,5-f][1,10]phenanthroline (L2), and 1,3-dithiole-2-ono[4,5-f][1,10]phenanthroline (L3), have been prepared and their structural, electrochemical, and photophysical properties investigated. Density functional theory (DFT) calculations indicate that the highest occupied molecular orbital of [Ru(bpy)2(L1)](PF6)2 (1) is located on the tetrathiafulvalene (TTF) subunit and appears ≈0.6 eV above the three Ru-centered d orbitals. In agreement with this finding, 1 exhibits three reversible oxidations: the two at lower potentials take place on the TTF subunit, and the one at higher potential is due to the Ru3+/Ru2+ redox couple. Complexes [Ru(bpy)2(L2)](PF6)2 (2) and [Ru(bpy)2(L3)](PF6)2 (3) exhibit only the Ru3+/Ru2+-related oxidation. The optical absorption spectra of all complexes reveal a characteristic metal-to-ligand charge transfer (MLCT) band centered around 450 nm. In addition, in the spectrum of 1 the MLCT band is augmented by a low-energy tail that extends beyond 500 nm and is attributed to the intraligand charge transfer (ILCT) transition of L1, according to time-dependent DFT calculations. The substantial decrease in the luminescence quantum yield of 1 compared to those of 2 and 3 is attributed to the reductive quenching of the emissive state via electron transfer from the TTF subunit to the Ru3+ center, thus allowing nonradiative relaxation to the ground state through the lower-lying ILCT state. In the presence of O2, complex 1 undergoes a photoinduced oxidative cleavage of the central C═C bond of the TTF fragment, resulting in complete transformation to 3. This photodegradation process was studied with 13C NMR and optical absorption spectroscopy. | ||||||||
 |
|
|||||||
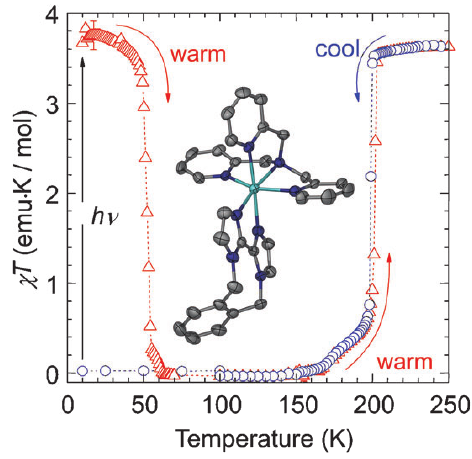
Three iron(II) complexes, [Fe(TPMA)(BIM)](ClO4)2⋅0.5H2O (1), [Fe(TPMA)(XBIM)](ClO4)2 (2), and [Fe(TPMA)(XBBIM)](ClO4)2 ⋅0.75CH3OH (3), were prepared by reactions of FeII perchlorate and the corresponding ligands (TPMA=tris(2-pyridylmethyl)amine, BIM=2,2′-biimidazole, XBIM=1,1′-(α,α′-o-xylyl)-2,2′-biimidazole, XBBIM=1,1′-(α,α′-o-xylyl)-2,2′-bibenzimidazole). The compounds were investigated by a combination of X-ray crystallography, magnetic and photomagnetic measurements, and Mössbauer and optical absorption spectroscopy. Complex 1 exhibits a gradual spin crossover (SCO) with T1/2=190 K, whereas 2 exhibits an abrupt SCO with approximately 7 K thermal hysteresis (T1/2=196 K on cooling and 203 K on heating). Complex 3 is in the high-spin state in the 2–300 K range. The difference in the magnetic behavior was traced to differences between the inter- and intramolecular interactions in 1 and 2. The crystal packing of 2features a hierarchy of intermolecular interactions that result in increased cooperativity and abruptness of the spin transition. In 3, steric repulsion between H atoms of one of the pyridyl substituents of TPMA and one of the benzene rings of XBBIM results in a strong distortion of the FeII coordination environment, which stabilizes the high-spin state of the complex. Both 1 and 2 exhibit a photoinduced low-spin to high-spin transition (LIESST effect) at 5 K. The difference in the character of intermolecular interactions of 1 and 2 also manifests in the kinetics of the decay of the photoinduced high-spin state. For 1, the decay rate constant follows the single-exponential law, whereas for 2 it is a stretched exponential, reflecting the hierarchical nature of intermolecular contacts. The structural parameters of the photoinduced high-spin state at 50 K are similar to those determined for the high-spin state at 295 K. This study shows that N-alkylation of BIM has a negligible effect on the ligand field strength. Therefore, the combination of TPMA and BIM offers a promising ligand platform for the design of functionalized SCO complexes. | ||||||||
Download this list in format RIS
 EndNote
EndNote  BibTex
BibTex  PDF XML
PDF XML Last update Friday December 08 2017